Explain How Atoms of Different Elements Are Different
It consists of three parts. The formation of a compound from its elements occurs through the combination of atoms of unlike elements in small whole number ratio.
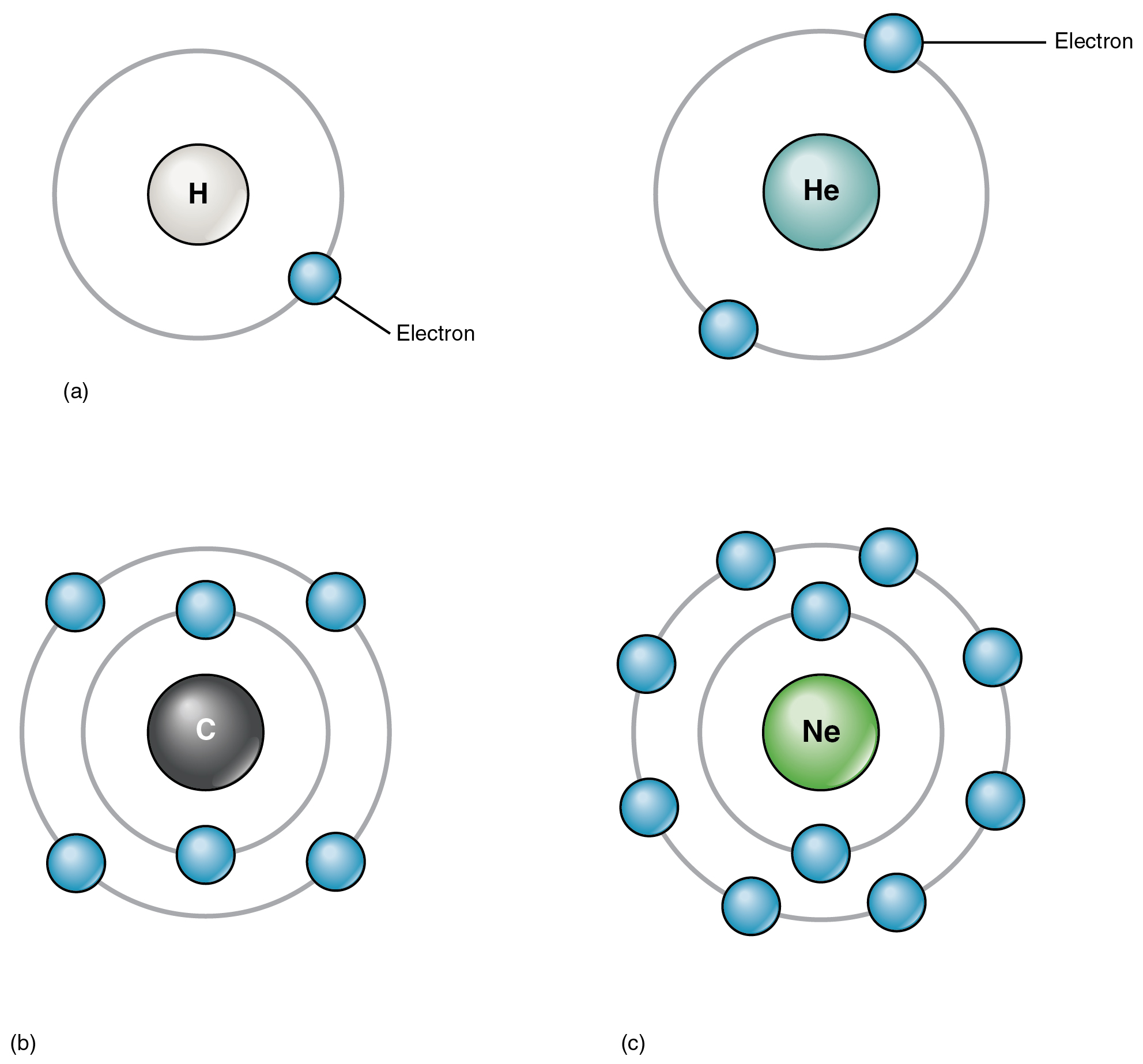
Elements And Atoms The Building Blocks Of Matter Anatomy And Physiology
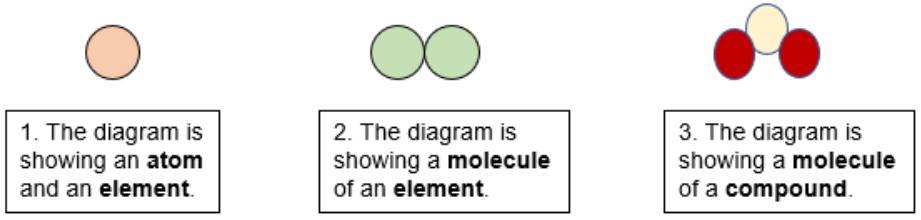
A molecule is formed when two or more atoms from the same element are chemically bonded whereas a compound is defined as the chemical bonding between atoms of different elements.
. As such the atom is the basic building block of chemistry. Different arrangements of atoms into groups compose all substances. Atoms of same element are exactly same and atoms of different element are different.
This tutorial introduces basics of matter. If you join two different chemical elements together you can often make a completely new substance. To be an element it can ONLY have ONE type of atom.
Al-Ghazâlî c10561111 was one of the most prominent and influential philosophers theologians jurists and mystics of Sunni IslamHe was active at a time when Sunni theology had just passed through its consolidation and entered a period of intense challenges from Shiite Ismâîlite theology and the Arabic tradition of Aristotelian philosophy falsafa. Each row and column has specific characteristics. Some elements occur in different forms such as graphite and diamond for the element carbon.
Knowledge series Chemistry Elements atoms and compounds Kevin Brace Page 6 of 24 Tasks. Thomsons Atomic Model Every atom is uniformly positive charged sphere of radius of the order of 10-10 m in which. There are more than 109 different elements known today.
8 During chemical combination atoms of different elements combine in small whole numbers to form compounds. The terms are often used interchangeably. Other sections include elements the periodic table reactions and biochemistry.
The rest consists of a positively charged nucleus of protons and neutrons surrounded. I Atoms of the same elements can combine in more than one ratio to form more than. Glue two atoms of hydrogen to an atom of oxygen and youll make a single molecule of water.
Physics Notes for Class 12 Chapter 12 Atoms Daltons Atomic Theory All elements are consists of very small invisible particles called atoms. The atoms of any element are alike but are different from atoms of other elements. Each element is placed in a specific location because of its atomic structure.
It also is the smallest unit of matter that has the characteristic properties of a chemical element. Build an Atom - PhET Interactive Simulations. The empirical formula is often the same as the molecular formula but not always.
3 Dan Kelly - Magic of Electrons - PLTW - Atoms 4. All matter is composed of atoms. For example the Periodic Table of the Elements could also be written as the.
Save teachers time and engage students with a new simpler interface. Different chemical elements have different kinds of atoms and in particular such atoms have different masses. Periodic Table of Different Types of Atoms.
Atoms may stick together in well-defined molecules or may be packed together in large arrays. Primary substances called elements build all the materials around you. Elements as Building Blocks The periodic table is organized like a big grid.
Atom smallest unit into which matter can be divided without the release of electrically charged particles. As with any grid the periodic table has rows left to right and columns up and down. Atoms of the same element are the same.
List five objects that are made from different materials eg. All matter is made up of atoms which are far too small to be seen directly through a microscope. FlexBook Platform FlexBook FlexLet and FlexCard are registered trademarks of CK-12 Foundation.
Water is a compound because its two different chemical elements joined together but its also a molecule because its made by combining atoms. The concept that atoms play a fundamental role in chemistry is formalized by the modern atomic theory first stated by John Dalton an English scientist in 1808. Atoms make up elements.
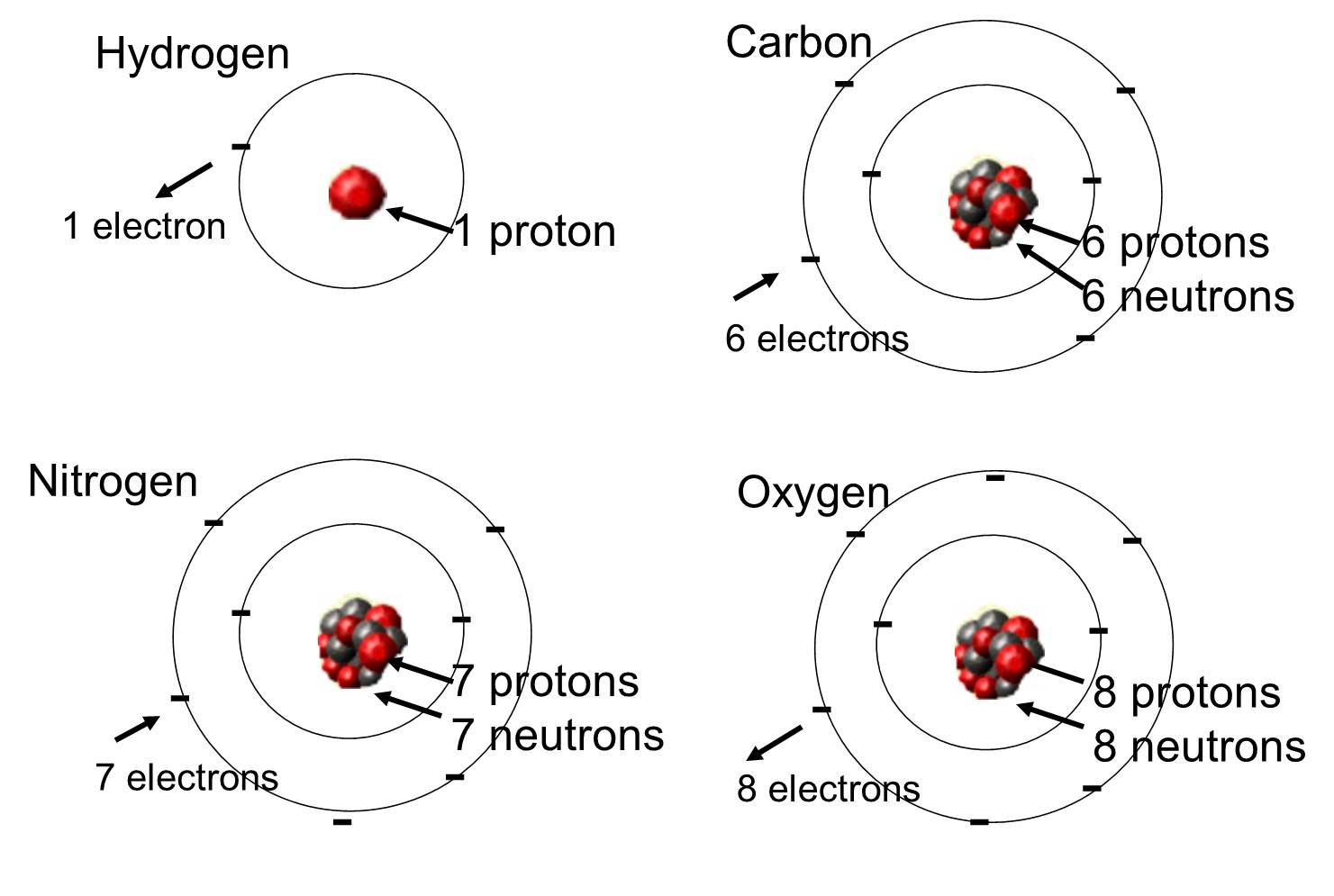
For example the molecule acetylene has molecular formula C 2 H 2 but the simplest integer ratio of elements is CH. Most of the atom is empty space. Atoms of different elements are different.
However different isomers can have the same atomic composition while being different molecules. 6 Atoms of different elements differ in mass size and chemical properties. On the bottom row list the opposite properties to those in the.
List all your objects in the left column. 7 The number and kind of atoms in a given compound is fixed. Atoms are indestructible and retain their identity in chemical reactions.
The elements are composed of atoms the smallest units that are characteristic of a particular element.
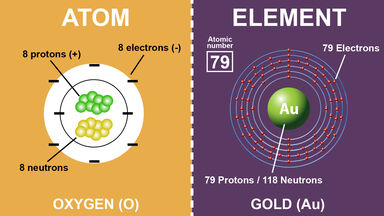
Difference Between Atoms And Elements With Examples

Understanding Atoms Elements And Compounds Lesson And Worksheets
Comments
Post a Comment